The UK may have made its official exit from the European Union (EU) on 31 January 2020; however, 31 December 2020 is the more important date for the €78bn EU cosmetics and personal care industry.
Although the UK is no longer part of the EU, existing EU legislation is currently still in place. This means that cosmetic products are still covered by the EU Cosmetics Regulation (1223/2009) until 31 December 2020, when the transition period expires.
At this point, all UK and EU cosmetic and personal care manufacturers and brands must be prepared for the UK to be outside of the EU regulatory framework and compliant with the future UK regulation.
Many companies are still not prepared for the changes that will come into force on 1 January 2021.
Some have had to refocus their efforts to deal with issues raised by the Covid-19 pandemic, such as furlough and huge fluctuations in supply and demand. Others have waited for the conclusion of the trade negotiations.
Whilst the outcome of these is likely to have significant consequences on the future of the UK, there is not expected to be any impact on the regulatory impact for cosmetics.
Regardless of the Future Trading Agreement, the UK legislation will take effect from 1 January 2021, replacing the existing EU regulation (1223/2009) within the UK.
Notable issues raised involve Cosmetic Product Notification Portal (CPNP) transfers, re-labelling, and the requirement for a suitable ‘Responsible Person’ (RP) as a legal entity to represent UK cosmetic businesses within the EU – or a suitable RP to represent EU businesses within the UK.
Taking responsibility
Every cosmetic product currently made available on the EU market must have a Responsible Person (RP). This legal entity must be “established within the Community”. From 1 January 2021, products intended to be sold in the UK and the EU post-Brexit will be subject to two different RP requirements: EU and/or UK RP.
Since a legal entity can only be established in one country, one entity will be unable to fulfil both requirements (it is possible that entities in Northern Ireland may be able to do this).
This is significant, as the RP must complete the EU CPNP or UK notification, compile and hold the Product Information File (PIF) and list its name and address on the product packaging.
In addition, the RP is responsible for ensuring the product is safe for use and must be able to discuss technical and safety issues with the relevant Competent Authority. This requires access to potentially sensitive information.
From 1 January 2021, all UK based RPs will be invalid in the EU. If a legitimate alternative is not arranged, then the EU importer will take on the role of RP. This means the importer would take on the associated liabilities and could request significant amounts of, potentially confidential, data to undertake its duties.
Since UK companies will no longer be legal entities within the EU post-Brexit, MSL Solution Providers has a wholly owned subsidiary business in Dublin, Ireland, which can take on this role.
Similarly, thanks to its Greater Manchester-based UK HQ, it will be able to act as the UK RP for EU businesses wishing to trade within the UK too. It is therefore able to offer a dual Responsible Person service, helping companies gain regulatory compliance and continuous access to both the EU and UK markets without having to deal with multiple service providers.
CPNP notifications
The EU CPNP (Cosmetic Product Notification Portal) intends to deactivate UK entities after the 31 December 2020, so it is crucial that products, which have previously been registered on the CPNP with a UK-based RP, are changed to an EU-based entity before 1 January 2021.
If this is not done in time, a full re-notification will be required. After this date, new products will not be able to be registered on the EU CPNP portal by a UK-based RP.
The UK intends to introduce the ‘UK notification database’, a similar registration system to the EU CPNP.
This will be available from January 2021 and every cosmetic product intended to be placed on the UK market must be notified on this database, with the support of a UK-based RP.
Re-labelling requirements
Product packaging labels will also need to be updated. Products with an EU RP will have two years from 31 December 2020 to add their UK RP address to labels.
In addition, ‘country of origin’ will also need to be added to labels, where they are currently exempt due to being made in the EU.
· Products sold in the UK, which are made in the EU, will need to have the specific country noted, eg ‘Made in Italy/France’;
· Products sold in the EU, which are made in the UK, will need to be labelled ‘Made in the UK’.
Different impacts on businesses
How these changes impact a business depends on two key factors, where its RP is based and where it sells or intends to sell its products. The answers to these two questions result in six scenarios.
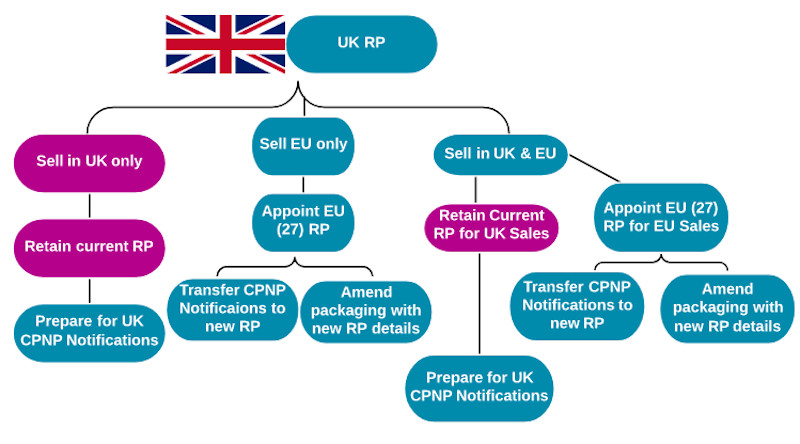
1) UK RP selling only into the UK
For businesses that currently have a UK RP and only sell products into the UK, there is no need to establish a new RP or update product packaging. They do, however, need to prepare for the new UK Cosmetic Regulation and, specifically, the UK notification database.
2) UK RP selling only into EU
Businesses must establish an EU presence as a legal entity, or, alternatively, mandate Responsible Person services to an RP within the EU, by 1 January 2021. EU CPNP notifications will need to be transferred or re-notified under the new EU RP details by this time.
Product packaging will also have to be updated to reflect the correct RP details and country of origin by 31 December 2020.
3) UK RP selling to the UK & EU
Having a UK RP and selling products in both the UK and into the EU is a challenging post-Brexit position. Businesses must prepare for the UK Cosmetic Regulation and UK notification database (see scenario one). They must also establish an EU RP to undertake EU requirements, including CPNP transfers and compliant packaging (see scenario two).
This can be done by setting up a company within the EU (with an EU address), or by employing a third party, EU-based RP to take on this role.
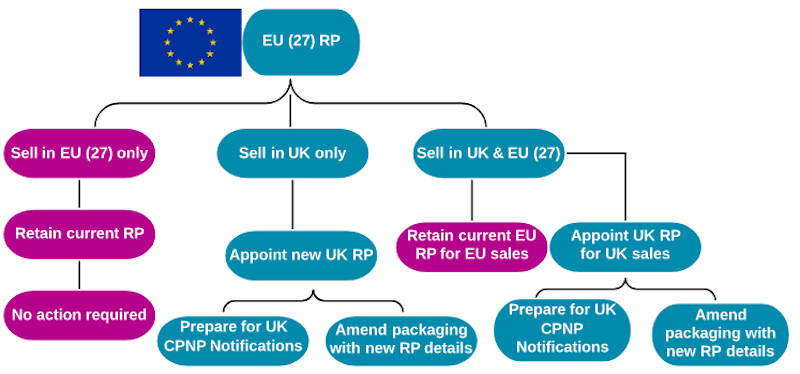
4) EU RP selling only into EU states
For companies with an EU RP, which only sell into EU countries, there will be no change to the regulatory situation. Unfortunately, these businesses will lose access to the UK’s cosmetic market – the third largest in the EU (€11.1bn in 2018), behind Germany (€13.6bn) and France (€11.3bn)[1].
5) EU RP selling only into the UK
For continued access to the UK market post-Brexit, EU businesses must establish a UK entity to act as their RP, or mandate Responsible Person services to a third-party RP within the UK.
EU CPNP notifications will become invalid in the UK, so businesses will be required to enter details on the UK notification database; there is a 90 day window to do this after 31 December 2020. Product packaging will need to be updated to reflect the correct RP details and country of origin by December 2022.
6) EU RP selling into the UK & EU
In this scenario, the EU requirements are satisfied by the existing EU RP. However, an additional UK RP will need to be appointed, and products will need to be entered on the UK notification database (see scenario five).
Product packaging will need to be updated to reflect the correct RP details and country of origin by December 2022.
Getting set for business post-Brexit
500+ SME manufacturers and an unknown number of brands make up the €11.1bn cosmetic and personal care industry in the UK[ii]. For these companies, and thousands more in the EU, time is running out to prepare for Brexit. It is vital that they understand the regulatory changes that come into force on 1 January 2021 and take action – or accept that they will be unable to trade in Europe (or vice versa) and will lose money.
Keep calm and keep on trading: Brexit case studies
As a specialist supplier of regulatory and Responsible Person services to the cosmetics and personal care industry, MSL Solution Providers has already helped hundreds of clients get ready for Brexit. Dapper Dan Ltd and Laneige (a brand of Amorepacific) are two such companies that have worked with MSL to prepare for Brexit in response to their specific needs.
Dapper Dan wanted to continue trading in both the UK and EU, but only had a UK-based RP. It did not want to set up another company in the EU, so mandated the role of its EU RP to MSL’s Irish subsidiary. MSL has handled all the relevant measures, including CPNP transfers and is working with the company on the required packaging changes. MSL will also be responsible for all UK RP duties.
Conversely, Laneige wanted to have a UK-based Responsible Person to cover post Brexit requirements for UK sales, so appointed MSL as the UK Responsible Person to carry out notifications on the UK Notification Database and all other UK RP duties.
Anna Ost, International Regulatory Affairs Director at Amorepacific Europe, comments: ‘Brexit has caused a certain amount of stress for the industry due to very late confirmation of regulatory requirements; it is clear that selling into the UK is made significantly more complicated by Brexit changes. We have been largely satisfied with MSL’s service during the preparatory phase. Its experts shared the future expected requirements for UK compliance in a very clear way, which allowed us to prepare, even though the UK Cosmetic Regulation is still not published.’
James May, Director at Dapper Dan Ltd, comments: ‘Brexit has caused a huge amount of uncertainty and stress for the industry. There has been so much confusion about what is changing and when.
“We have been so impressed with MSL’s service. Their experts explained in clear, easy-to-understand language what we needed to do to remain compliant and helped every step of the way.”
References
1. https://www.statista.com/statistics/382100/european-cosmetics-market-volume-by-country
2. https://www.statista.com/statistics/382100/european-cosmetics-market-volume-by-country

