In 2026, beauty retailers across Europe and Canada will face a synchronised compliance cliff, as sweeping new regulations governing cosmetic ingredient disclosure come into force. The simultaneous enforcement of the updated INCI Glossary under Commission Implementing Decision (EU) 2025/1175 and the expanded fragrance allergen disclosure rules under Commission Regulation (EU) 2023/1545, alongside Canada’s phased implementation under SOR/2024-63, marks a turning point for ingredient transparency and supply chain accountability.
Historically, cosmetic regulation focused primarily on the safety of ingredients—banning carcinogens or restricting preservatives. The new wave of regulation, however, focuses on informational safety. The premise of Regulation (EU) 2023/1545 is not that the newly listed 56 allergens are unsafe for the general population, but that they pose a specific risk to sensitised individuals who have a "right to know". This philosophical shift places the burden of granular data disclosure squarely on the manufacturer. It is no longer sufficient for a product to be safe; its safety profile must be fully transparent, legible, and accurate down to the level of trace isomers in a complex essential oil blend.
Ket Dates:
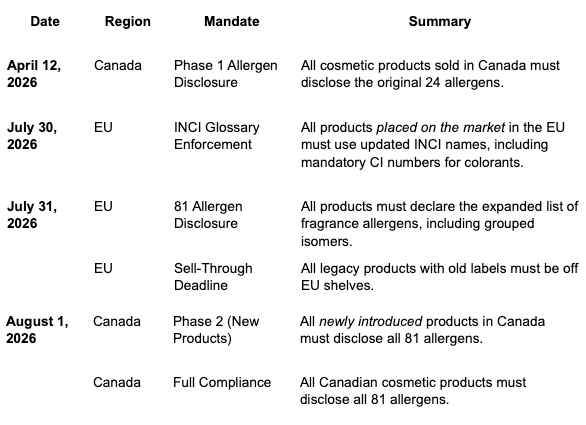
Operational Implications for Beauty Retailers, Specialty Stores, and Department Stores:
Beauty retailers are being called upon to audit their inventories, enforce brand partner compliance, and establish centralised ingredient governance protocols.
To achieve this:
- Brands must provide updated ingredient lists in official INCI format, not proprietary or outdated nomenclature or translations. Retailers should request allergen disclosures based on the new 81-substance list.
- Allergens must be flagged and aggregated correctly—particularly isomer families such as “Rose Ketones” and “Citral,” which must be summed across multiple ingredients to determine threshold compliance.
- Ingredient data must be continuously updated, verified, and made visible at the point of sale—on pack and online.
Beauty Retail Solutions: From Manual Cleanup to Automation
To avoid manual overload and regulatory exposure, automated systems are now essential. One such solution are the Transparency Features form Inference Beauty, mainly the INCI Explainer, a retailer-ready platform that streamlines INCI compliance across complex inventories:
- 147,166 unique products (EAN-level)
- 2,647 brands
- 110+ product categories spanning skincare, color cosmetics, body care, and fragrance
- 60,000+ ingredients classified in official INCI format
- 100+ attributes per ingredient, including source, utility, and claims like vegan, Microplastic-Free and as for the deadlines also allergen; markings.
Retailer Benefits Include:
- Complying with Canadian and EU law across all listings
- Reducing in-house regulatory workload
- Minimising customer service queries related to allergens and ingredients
- Centralising brand onboarding with structured data controls
- Avoiding customer migration to third-party databases (e.g., CodeCheck, Yuka) by offering transparent data in-platform
"2026 will be remembered as the year ingredient transparency stopped being optional. This is a decisive moment for the industry—and a huge opportunity for retailers to lead with clarity and trust. At Inference Beauty, we've spent years building the infrastructure to make ingredient data actionable, compliant, and customer-ready at scale. With the Ingredient Explainer, we’re not just helping our partners meet the new laws—we’re helping them build a smarter, more transparent beauty future."
— Estella Benz, CEO, Inference Beauty

Next Steps for Beauty Retailers
Inference Beauty recommends the following compliance roadmap for 2026:
- Q4 2025–Q1 2026: Initiate brand outreach, mandate INCI-only submissions, conduct internal INCI scrub
- Q1–Q2 2026: Update e-commerce ingredient displays, validate allergen data, prepare for July deadlines
- Q2 2026: Begin soft rollout of new labelling across SKUs to meet “Placing on the Market” requirements
- 2027–2028: Monitor retail sell-through, enforce removal of non-compliant legacy products
Legal Resources:
European Union
- Commission Regulation (EU) 2023/1545: Amending Regulation (EC) No 1223/2009 as regards labelling of fragrance allergens in cosmetic products. Relevance: The definitive list of 81 allergens and grouping rules.
- Commission Implementing Decision (EU) 2025/1175: Establishing a glossary of common ingredient names for use in the labelling of cosmetic products. Relevance: The mandatory dictionary for ingredient names effective July 30, 2026.
- SCCS/1459/11: Opinion on Fragrance Allergens in Cosmetic Products. Relevance: The scientific rationale and background on pre/pro-haptens.
Canada
- SOR/2024-63: Regulations Amending Certain Regulations Concerning the Disclosure of Cosmetic Ingredients. Relevance: The legal text enforcing the April 2026 and August 2026 mandates.
- Health Canada Industry Guide for the Labelling of Cosmetics: Relevance: Practical guidance on the phased implementation.

