In Ayurveda the grains of wheat, barley and rye have been used since time immemorial for the treatment of topical hyperpigmentation. Some 3,000 years later it was shown that this property boils down to partial inhibition of tyrosinase – the enzyme behind melanin formation – by azelaic acid.
Azelaic acid, a saturated dicarboxylic acid chemically identified as 1,7-heptanedicarboxylic acid, has been shown to be the real workhorse. Azelaic acid exhibits bactericidal properties and this has resulted in the application of azelaic acid for the treatment of acne vulgaris. Its anti-inflammatory properties have also resulted in the application of azelaic acid for the treatment of rosacea. It also shows interesting synergies with other physiologically active ingredients, such as for hair regrowth products, and may act as a standaalone ingredient as well.
Synthesis of azelaic acid
Commercial production of azelaic acid from botanical resources is commercially not feasible. At present, azelaic acid is produced by oxidation of commercially available oleic acid. The oleic acid used originates from sunflower oil or from milk thistle oil (Marian thistle, Silybum marianum), or a mixture thereof. This is a mixture of a number of related fatty acids, both unsaturated and saturated, with a chain length of 12-24 carbon atoms. The unsaturated fatty acids may have the double bond at different positions; cis-Δ9,10- octadecenoic acid is the predominant fatty acid; in practice also di- and tri-unsaturated fatty acids are present, more particularly linoleic and linolenic acids. Traditional grades of azelaic acid are therefore accompanied by other dicarboxylic acids, more particularly glutaric acid, adipic acid, pimelic acid, suberic acid and sebacic acid, and are usually offered as 90-95% purity grades.
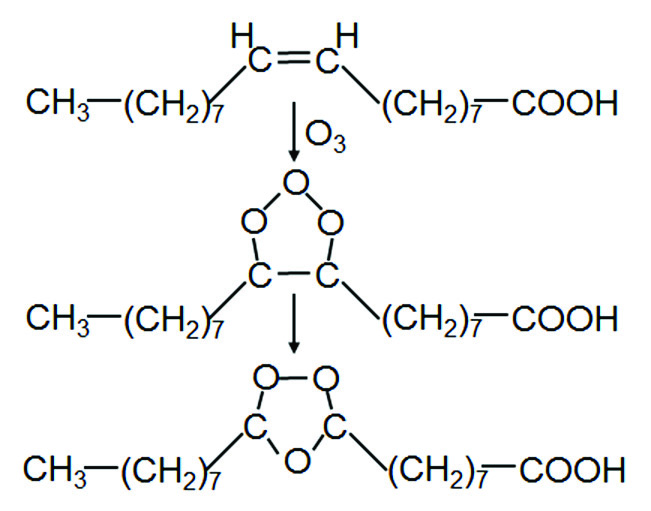
The traditional route for the production of azelaic acid is by ozonisation of oleic acid. Addition of ozone to the double bond of the fatty acid leads the formation of a molonozide, a five-membered cyclic product containing three oxygen atoms in the 1-, 2- and 3-position. Molonozides are unstable and rearrange swiftly into the corresponding ozonozide, whereby the oxygen atoms are positioned in the five–membered ring in the 1-, 2- and 4-position.
Upon hydrolysis of the ozonozide, followed by oxidation, a carboxylic acid and a dicarboxylic acid will be formed. Thus, ozonolysis of Δ9,10-octadecenoic acid results in the formation of azelaic acid and nonanoic acid. Ozonolysis of petroselinic acid (cis-Δ6,7-octadecenoic acid) results in the formation of adipic acid and lauric acid. Ozonisation is a process that is difficult to control. The reaction frequently runs out of control and a hazardous explosion may result. It is interesting to note that conversion of the ozonozide may also be accomplished using a catalase[1], resulting in less severe reaction conditions.
An alternative reaction pathway is the conversion of the double bond into the corresponding epoxide. This is best done using a peracid, such as peracetic acid or (preferably) performic acid[2]. Formic acid and acetic acid are, in the presence of hydrogen peroxide, in equilibrium with the corresponding peracid; the explosion limits of equilibrium mixtures of formic and/or acetic acid are well-known and can be properly controlled. Oxidation of the epoxide subsequently results in cleavage of the C-C bond whereby a carboxylic acid and a dicarboxylic acid are formed. The peracid route is a safe route, contrary to the ozonisation process. Purification of crude azelaic acid, however, is a tedious process, and is usually considered to be the intellectual property of the producing companies. An example of such a purification process has been described by Gaige, McVay & Ewbank and Öhlschlaeger & Rodenberg[3].
Currently a 99+% grade azelaic acid is commercially available, enabling the product to be properly registered for both cosmetic and pharmaceutical applications.
Chemical and structural formula of azelaic acid
Activity vs acne vulgaris
Azelaic acid is detrimental for particular organisms such as Propionibacterium acnes and Staphylococcus epidermidis. These reside in the sebaceous glands present in the dermis[4], and are held responsible for acne vulgaris. Azelaic acid acts specifically on P.acnes and S.epidermidis, and does not affect most other native organisms.
It also decreases the production of keratin, required for the growth of P.acnes; P.acnes destroys the wall of the sebaceous glands to obtain the required nutrients, more particularly proteins that are hydrolysed into the corresponding amino acids[5].
Medications for acne include benzoyl peroxide, salicylic acid, α-hydroxy acids, retinoids, antibiotics (doxycycline, clindamycin), nicotinamide (vitamin B3) and keratolytic preparations[6]. Benzoyl peroxide and salicylic acid especially are currently under scrutiny. The FDA issued the statement: “The Food and Drug Administration (FDA) is warning that the use of certain acne products containing the active ingredients benzoyl peroxide or salicylic acid can cause rare but serious and potentially life-threatening allergic reactions or severe irritation. An active ingredient is the component that makes the medicine effective against the illness or condition it is treating.”[7].
Side effects are virtually absent with azelaic acid. Azelaic acid is believed to function on the basis of its antimicrobial activity and/or normalisation of keratinisation (the process by which epithelial cells mature as they move towards the skin surface and are then desquamated). The European Union considers azelaic acid as a cosmetic ingredient that can be applied without concentration restrictions. In the US, azelaic acid is FDA approved for the treatment of acne and rosacea. A variety of products containing 10-25% azelaic acid are commercially available.
The effect of a topical acne treatment made with azelaic acid on the transmembrane pH gradient (ΔpH) of P.acnes and S.epidermidis was studied in vitro at external pH values found on human skin (pH4-6). The results indicate that the antibacterial activity of azelaic acid is associated with the perturbation of intracellular pH of the organisms. The effects of topically applied azelaic acid were studied with 47 individuals (12 with normal skin, 15 with seborrheic skin and 20 suffering from acne)[8]. Topical application of a 20% azelaic acid cream significantly reduced the number of lesions with the acne patients, but failed to induce clinically detectable changes in normal or seborrheic epidermis.
Complementary investigations showed that azelaic acid treatment did not induce changes in sebum composition, excretion rate or the size of sebaceous glands, but epidermal keratinisation markedly changed. The epidermis of the acne-suffering individuals showed a marked reduction of the thickness of the stratum corneum (the horny layer) and normalisation of filaggrin distribution (filaggrin is a protein that assures proper water management in the horny layer).
In vitro, azelaic acid exerted marked time- and dose-dependent anti-proliferative cytostatic effects on cultured keratinocytes, with a 50% inhibitory dose of 20mMol. This is indicative of the anti-keratinising properties of azelaic acid, displaying anti-proliferative cytostatic effects on keratinocytes and modulating the early and terminal phases of epidermal differentiation[9].
Treatment of rosacea
Rosacea is sometimes named “the curse of the Celts”[10]. It mostly affects people from northwestern descent. It is a chronic condition characterised by facial redness (erythema). Without treatment rosacea worsens in time; treatment with antibiotics is counter-effective and their use is being discouraged. The cause(s) of rosacea are not yet clear, although it has been concluded that allergies may play a significant role. Also weather conditions (high sun intensity, cold/warm weather, wind, frost) may contribute to episodes of flushing. Also a disturbed balance of the intestinal flora is considered as a cause for rosacea.
Azelaic acid has been demonstrated to be effective for the treatment of rosacea. It is available as a 20% cream or a 15% hydrogel. It reduces inflammatory lesions and erythema in rosacea patients and also inhibits neutrophilic reactive oxygen species (ROS). In the neutrophil system, azelaic acid inhibits the ROS formation in a dose-dependent manner, markedly decreasing the number of free radicals. In the xanthine-xanthine oxidase system, none of the ROS generated was decreased by any dose of azelaic acid, indicating that azelaic acid does not scavenge generated ROS, but rather inhibits cell metabolism, possibly by decreasing enzymatic activity within the cell membrane. In a study by Akamatsu[11] it was found that NADPH oxidase activity on the neutrophil surface membrane, which mediates the neutrophilic production of most ROS, is effectively inhibited by lower concentrations of azelaic acid.
Skin whitening
Azelaic acid is also used for the treatment of melasma, lentigo maligna and other disorders of hyperpigmention[12,13]. Azelaic acid has been reported to be effective for hypermelanosis caused by physical or photochemical agents, and lentigo maligna melanoma, as well as other disorders characterised by abnormal proliferation of melanocytes. Its mechanism of action is to inhibit DNA synthesis and mitochondrial enzymes, thereby inducing direct cytotoxic effects toward the melanocyte[13].
Acne lesions are frequently darker in colour compared with the surrounding skin. Azelaic acid can effectively be used for this post-inflammatory hyperpigmentation, believed to be caused by ROS. Free radicals are believed to contribute to hyperpigmentation and azelaic acid acts by reduction of the free radical production. Topically applied azelaic acid does not result in depigmentation of normal pigmented skin and freckles. Although at present there is still insufficient proof, the specificity may be attributed to its selective effects on abnormal melanocytes rather than normal melanocytes.
Hair growth
Azelaic acid is a well-known inhibitor for 5α-reductase: the enzyme that converts testosterone into 5α-dihydrotestosterone, a major cause for alopecia areata and hair loss. Due to the relative absence of dihydrotestosterone, regression of the hair follicle will take place (the catagen stage) and subsequently hair generation will take place (the anagen stage). This enables the use of azelaic acid for hair regrowth.
Unfortunately the number of clinical studies is limited; the pilot study dates back to 2005[14]. This study was performed to evaluate the effectiveness and safety of azelaic acid on patients suffering from alopecia and/or alopecia areata. The results using azelaic acid were compared with the effectiveness of anthralin (1,8-dihydroxy-9- oxoanthracene), a drug prescribed to treat psoriasis and frequently used for the treatment of alopecia areata. It was concluded that the performance of azelaic acid is comparable with the results obtained with anthralin for hair (re)growth.
Also Zn2+-salts are inhibitors for 5α- reductase; the efficacy of zinc sulphate, for example, was significantly increased with the simultaneous use of pyridoxine hydrochloride (vitamin B6). Pyridoxine hydrochloride did not result in increased efficacy of azelaic acid. However, the combination of the three products at low doses showed a 90% reduction in 5α-reductase activity[15].
Conclusions
Azelaic acid is best defined as a cosmeceutical ingredient, ie it shows both cosmetic and pharmaceutical properties. The performance of azelaic acid exhibits superior performance for the treatment of acne vulgaris, rosacea and melasma, and also shows high performance for hair (re)growth and skin lightening by means of enzyme inhibition. The toxicological properties of azelaic acid are superior compared with the current pharmaceutical products used for the treatment of acne vulgaris or rosacea; the side effects of azelaic acid are minimal and can be mastered rather straightforwardly, using commercially available osmolytes.
Authors
Elzbieta Brand-Garnys and Dr Hans Brand, Elzbieta Cosmetics
References
1. M.Ullrich, P.Hannen, M.Roos, US 2013,0078685 A1, assigned to Evonik.
2. D.Swern, Chemical Reviews 45,1,(1949).
3. D.G.Gaige, K.R.McVay, E.L.Ewbank, US 2003,0032825 A1, Method for purifying azelaic acid; H.F.Öhlschlaeger & H.G.Rodenberg, US 3,402,108 A, assigned to Emery Industries.
4. D.Thiboutot, J.Drugs Derm., 7,13,(2008).
5. C.C.Zouboulis, Acne and sebaceous gland function, Clinics in Dermatology, 22,360,(2004).
6. M.Ramos da Silva, S.C.Carneiro, Acne vulgaris: Review and guidelines. Dermatology nursing / Dermatology Nurses' Association 21,63,(2009).
7. FDA (US Food & Drug Association), Topical Acne Products Can Cause Dangerous Side Effects (http://www.fda.gov/ForConsumers/Consumer Updates/ ucm402441. htm).
8. http://toxnet.nlm.nih.gov/cgibin/ sis/search2/r?dbs+hsdb:@term+@rn+@re l+123-99-9.
9. A.Mayer da Silva et al, Acta Derm.Venereol.Suppl., 143,20,(1989). 10. U.Wollina, S.B.Verma, J.Cosm.derm.8,234,(2009).
11. H.Akamatsu, J.Komura, Y.Asada, Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch.Dermatol.Res., 283,162,(1991).
12. P.E.Grimes, Arch.Dermatol., 131,1453,(1995).
13. Q.H.Nguyen, T.P.Bui, Azelaic acid: pharmacokinetic and pharmacodynamic properties and its therapeutic role in hyperpigmentary disorders and acne, Int.J. Dermatol., 34,75,(1995).
14. A.J.J.Wood, V.H.Price, N.Engl.J.Med., 341,964,(1999).
15. D.Stamatiadis, M.C.Bulteau-Portois, I.Mowszowicz, Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid, Br.J.Dermatol., 119,627,(1988).
