Institutes Dr. Schrader have set standards in the efficacy testing of sunscreen products.
During the last decades the knowledge about the importance of a comprehensive protection against short-wave solar radiation (UV-B and UV-A) has rapidly increased in the population worldwide.
Required by dermatology, this led almost inevitably to a continuous improvement of sun screen products. Self-evident SPF-testing methods became more and more important. Independent of the type of testing, UV-B protection or UV-A protection, in vivo or in vitro, results have to be reproducible. For the developer of modern sunscreen products it is extremely important to get quick and reliable test results, in order to be able to adapt the product quality to the changing market situation.
SPF
Already in the 1970 years Institutes Dr. Schrader started with the scientific in vivo testing of sun protection. Existing test methods were questioned and improved by permanent intensive in-house research.
Numerous publications and awards in the field of sun protection testing document this successful work. The participation in national and international task forces and conferences as well as regular contributions in ring studies have become self-evident for Institutes Dr. Schrader over the last four decades of SPF research.
The investigation of thousands of products in the field of SPF and UV-A protectionin vivo and in vitro leads to a constant high level of customer satisfaction. Highest technological expertise created an outstanding know-how that has yet to be matched.
Externally certified Sun Simulators create a unique quality of measurements. On top each sun simulator is constantly monitored by an in-house Medical Device Technology Department. In combination with well trained technicians and modern air condition technology, clients can always trust in precise, reproducible and legally robust results.
Independent of the chosen method: International SPF Test Method 2006 (COLIPA CTFA CTFA-SA-JCIA U.S.), ISO 24444:2010 (E), FDA Final Rule 2011 or AS / NZS 2604, all globally established methods in the field of SPF testing are offered routinely.
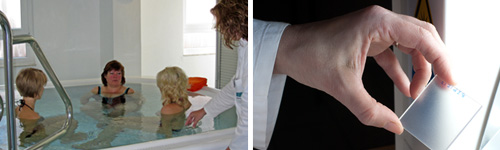
Water Resistance
For the determination of water resistance the cost-saving shower method is offered and frequently used during sunscreen product development. Of course, COLIPA Spa Pool method from 2005, Spa Pool method defined by FDA Final Rule 2011 and the testing according to AS/NZS 2604 are implemented as well.
The Spa Pool method is optimised in regard to fluid dynamics and jet geometry by a special designed spa pool. This results in a uniform water flow that prevents a direct impact on the test area, in order to meet all standard requirements.
Huge capacities allow shortest timings for customers.
UVA
In the field of UV-A protection of sunscreen products, customers of Institutes Dr. Schrader have the choice between in vivo and in vitro tests in accordance with the current methods.
For in vivo studies the PPD-determination according to ISO 24442 or FDA 2011 is used – nowadays a worldwide standard based on JCIA STD 1999.
For in vitro studies Institutes Dr. Schrader are able to meet the requirements of all published methods. The extraordinary precision of the used spectrometer allows measurements up to the highest absorption levels. All widely used in vitro methods in Europe like ISO 24443 / COLIPA 2011 (COLIPA Ratio) or Boots Star Rating (Revision 2011) and the in vitro determination of UVA protection according to the FDA Final Rule 2011 or AS / NZS 2604 are implemented.
Decades of experience and the readiness for the constant investment into the know-how of our technicians, as well as the use of most modern technologies are a guarantor for your precise SPF results.
Quality through Experience – makes your success.