The determination of skin sensitisation potential is one of the most important toxicological endpoint in the development and evaluation of ingredients used in fragrance, cosmetic and personal care products.
Since the total animal testing ban in March 2013, in vitro skin sensitisation tests are still one of the current hot topics in the world of cosmetic ingredients evaluation. During the last few years numerous of in vitro tests have been developed.
The approach used for in vitro sensitisation tests development is based on the AOP concept (Adverse Outcome Pathway) summarised below:
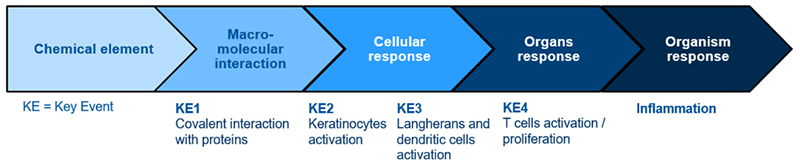
AOP concept relies with the analysis of every step of the mechanistic reactions involved in the sensitisation phenomenon, from the chemical structure and properties of the potential sensitiser, to the organism response.
It allows to determine 4 major events names “Key Event”, numbered from 1 to 4. The sensitisation mechanism cannot be summarised with only one Key Event, the aim is to cover the maximum of Key Event.
Among the in vitro alternative methods developed we can cite the followings:
- KE1: DPRA
- KE2: KeratinoSens, LuSens, SENS-IS, IL18 assessment
- KE3: h-CLAT, U-SENS, GARD, VitoSens, IL8-Luc assay
KE4 being at the level of organ, there is no in vitro test for this step.
Among them 5 are fully validated and are covered by an OCDE Guideline: DPRA (OECD 442C), KeratinoSens and LuSens (OCDE 442D) and h-CLAT, U-SENS and IL8-Luc assay (OECD 442E). For SENS-IS and GARD an external validation has been completed and is under review at the ESAC.
As previously discussed, due to a mechanistic complexity of the final endpoint and the fact that no specific step or reaction has been identified for the determination of the potency of an allergen, an Integrated Testing Strategy (ITS) is needed for the skin sensitisation hazard identification and classification.
However, there is no official or recommended testing strategy and we can see many individual strategies as described in the annex I of the OECD No 256 document.
ITS approaches have to use a combination of individual alternative assays such as in silico, in chemico and in vitro methods and take into account the validated (or ongoing) methods that are already in use by cosmetic industries and CROs. Ideally, the ITS should aim to evaluate the 3 major Key Events: KE1, KE2 and KE3.
The most used are the DPRA, the KeratinoSens (or LuSens), the h-CLAT (or U-SENS).
Based on the predictivity and the technical availability, as a testing strategy, the approach often used is a "2 out of 3" integrated testing approach to skin hazard identification.
3 tests, i.e. DPRA, KeratinoSens or LuSens and h-CLAT or U-SENS are performed together in combination and 2 of 3 tests must be matching for rating, e.g. to predict skin sensitisers 2 of 3 tests must be positive.
The 3 tests can be performed sequentially and some companies adopted the strategy to stop at the first one if the result is positive (sensitiser).
Due to the potential complexity of cosmetic ingredients (i.e. UVCB) the DPRA is often not applicable and a fourth test is then use in the ITS. In that case the most currently used test is the SENS-IS.
Other strategies can therefore be used, still with the "2 out of 3" approach, such as SENS-IS + h-CLAT + KeratinoSens or DPRA + SENS-IS + h-CLAT.
One can expect that more method will be available and validated in the near future and mainly that EURL ECVAM will take the leadership for the development of the skin sensitisation ITS and for its implementation in EU legislation in order to help the manufacturers in safety evaluation and classification.
The data obtained using these different in vitro tests and ITS for hazard identification have to be part of a Weight of Evidence Approach (WOE) for assessment of sensitising potential. This also includes chemical/physical characteristics of the product, experimental data of historical (before March 2013) in vivo tests, HRIPT, and human data.
Then a risk assessment could be made based on the QRA approach. It introduces exposure information to the chemicals and its sensitisation potency. The in vitro validated methods are able to provide quantitative potency data, but so far, only the SENS-IS test proposes a potency class estimation.
Whatever your project or your product is, the sensitisation potential of an ingredient is a critical element of the safety assessment and IDEA Lab can help you to establish the right testing strategy according to the type of sample and in combination with the other toxicological tests.
Frédéric NUNZI, PhD
Doctor in Cell Biology and Microbiology / Head of IDEA Lab
Eurotox Expert (Regulatory and in vitro Toxicology)
f.nunzi@groupeideatests.com
References
- McKay, C. et al., (2013) From pathways to people: applying the Adverse Outcome Pathway (AOP) for skin sensitization to risk assessment. Altex 30, p473-486.
- OECD No 256 – Series on Testing and Assessment - Annex I: Case Studies to the Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to be used within Integrated Approaches to testing and Assessment (IATA) for Skin Sensitization. Oct. 2016.
- Chemical Substances of Unknown or Variable Composition, Complex Reaction Products and Biological Materials.
- Henkel AG & Co. KGaA (2016) Safety evaluation of cosmetic ingredients regarding their skin sensitization potential. Cosmetics, 3, 14.
- Basketter, D.; Safford, B. Skin sensitization quantitative risk assessment: a review of underlying assumptions. Regul. Toxicol. Pharmacol. 2016, 74, 105–116.
- Corsini E. et al., (2016) Alternative approach for potency assessment: in vitro methods. Cosmetics, 3, 7.




