Too often, the cosmetics industry evaluates a company's new product introduction (NPI) process by the ability to go to market effectively and timely.
With the global expansion of the cosmetics industry, the burden of increased regulations, coupled with the lack of relevant content and efficient technology, has caused serious delays in taking new products to market quickly without compliance issues.
When it comes to new productivity-enhancing methods and technologies, two of the greatest advantages that are often overlooked are the increased number of available hours for teams and the significant financial savings that come with a streamlined system.
Unintended costs
In this industry, we are all too familiar with the hidden difficulties and costs of taking a product to market, such as:
- Region-specific compliance requirements;
- Gathering required documentation from suppliers;
- Balancing the need for speed to market while remaining compliant;
- The cost to re-label products;
- The cost of air-freighting reformulated products;
- And delays in getting to market with a competitive product.
In fact, it is all too common to accept that these costs happen, and to plan to react to these issues instead of taking a proactive approach.
Without the use of technology, the act of streamlining a complex process into sequential and manageable steps gets increasingly overwhelming. When this occurs, mishaps are made.
The list of excuses when companies face delays in getting to market quickly and compliantly include:
- Inability to find documentation;
- Lack of information from suppliers;
- Use of antiquated, manual tools;
- Inability to access up-to-date formulation information;
- Inflexible, non-integrated processes;
- And delays by regulatory department.
And while implementing a technical solution can solve almost all your issues and risks, as shown in figure 1, what often gets overlooked is the gain in productivity and work hours, and the significant financial savings.
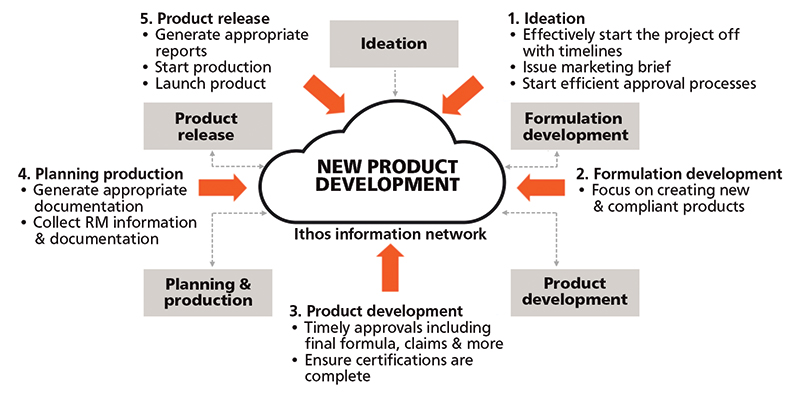
Figure 1: New product introduction
Stage-gating the NPI process
To have an effective NPI process, it is necessary to have a guide with a sequence of tasks and appropriate checks in place to allow for visibility to contain mistakes and confront problems.
One approach is the stage-gating process; there is typically a series of five stage-gates that occur (figures 1 and 2).
The issue within the industry is the common use of manual tools along this NPI process. Frequently, each team's individual workflows are not integrated, and delays and costs are not clearly tracked.
In many cases, tasks are dependent upon required regulatory documentation and can't be started until approval is granted.
This leads to companies operating in a reactive mode with extraneous costs for legal, rework and repairs, let alone the wasted time for employees.
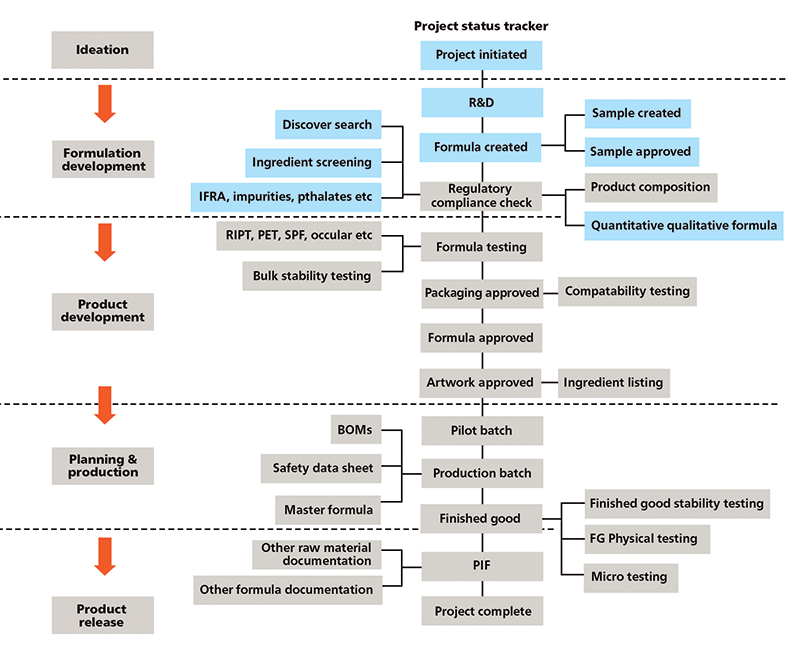
Figure 2: Five stage-gates to NPI
Automating the NPI process
The combination of a stage-gating process with technology allows for a proactive approach to the NPI process, resulting in streamlining, fewer hours wasted and greater visibility.
- Approval processes that used to take weeks are now automated and the overall cycle time is reduced by 50-75%;
- Marketing can create artwork knowing that the formula was approved by regulatory;
- Management can see which deliverable has been holding up the process; and
- Supplier documentation can be located quickly and attributes easily queried.
Automating the NPI process allows companies to gain significant savings in expenses, reduce time to market, and better use the hours saved via being more efficient.
Not only do employees save time completing remedial tasks, avoid duplicated efforts and quickly retrieve necessary information, but time savings allow employees to prioritise aspects such as coming up with innovative solutions.
Without the use of technology, the act of streamlining a complex process into sequential and manageable steps gets increasingly overwhelming
A deeper dive into NPI
When taking a closer look at each part of the stage-gating process, there are many things that can be done to make it simpler:
1. Ideation
- Challenges: Effectively starting the project with a timeline, issuing a marketing brief and starting efficient approval processes.
- Actions: Start off strong with an automated system and initiate workflows to see what steps need to be carried out, including raw material qualifications.
- Ensure that information is up-to-date from the start and centralised in order to effectively make decisions and approvals, share between departments and avoid mistakes later on.
2. Formulation development
- Challenges: Formulation developers need to focus on creating new and compliant products, yet are pressed for time. Approvals must be completed in good time yet accurately. What can be shipped to which country with the least amount of work?
- Actions: Screen products for compliance early, so you can easily see which ingredients in your formulas are compliant before developing products for multiple regions. Involve regulatory earlier in the process to proactively note when restrictions are present.
3. Product development
- Challenges: Timely approvals including final formulas, claims and more. Documenting the approval process and pinpointing which information is still needed.
- Actions: Automate formula approvals and ingredient lists to reduce manual work and extended approvals. Use a centralised location to store information and documentation necessary for approvals.
4. Planning & production
- Challenges: Generating acceptable safety data sheets. Collecting specs and supporting documentation for raw materials and having current documentation.
- Actions: Complete documentation with the click of a button and easily see the documents you have for each raw material or formula.
5. Product release
- Challenges: Finalising everything needed for production.
- Actions: Generate and share PIF reports based on formula information and output in a dossier that can be shared via email.

An implementation road map
In order to automate your NPI stage-gating process with technology and content, it is important to create a road map of what you want implemented.
Below are a few examples of what a compliance reporting system's design plan phases might look like.
Phase 1
Establishing a centralised repository of regulatory frameworks and managed content on ingredients for reviews and reporting.
Understanding which jurisdictions your product is going to be sold into, assuring you have up-to-date regulatory content that can be applied to the product's ingredients.
The process today is probably manual at your company and can delay product releases. Regulatory needs an efficient tool to select a formulation and evaluate it against content for multiple jurisdictions.
Phase 2
Establishing a supplier portal for centralising, collecting and accessing raw material and product related data and documentation.
Chances are that information and documentation is either siloed in different business systems or non-existent. Create one 'record of truth' that all stakeholders can quickly access.
Phase 3
Refine stage gates 1 and 2 with workflows and dashboards so that information is shared, reviewed and acted on in a timely manner, with visibility to management.
This refining process should include integration with your formulation management system, PLM or ERP.
Relevant data resides in all of these systems and needs to be aggregated into a single, user-friendly dashboard/report view.
Return on investment
Doing a business review of work hours, issues and challenges demonstrates how implementing an automated system for product development could give you significant reductions in time-to-market while freeing up significant person-hours for your team.
Within each stage, from ideation to product release and with every deliverable and approval, a streamlined process reduces the costs associated with inaccuracies and inefficiencies.
Glaring facts include how many people work in each department and how often they repeat processes, work on redundant tasks and waste time researching suppliers.
Another takeaway is: what is my cost of doing nothing and standing still?

Ithos Global is the Platinum
Sponsor of the Cosmetics
Business Regulatory Summit 2018
If someone told you that you could get to market 75% faster by making fewer mistakes, instantly, accessing real time data and having 35% more productive work hours from your existing staff without hiring one new person, you would be doubtful.
If someone told you that you could save more than US$1m per year in costs while boosting your reputation, timeliness and data accuracy, you would be even more sceptical.
Ithos Global conducts business reviews with its prospective customers to show a planned ROI – it is then held to that level of return as content and technology is implemented.
Solving information deficiencies is critical for successful new product introductions. By incorporating tools that foster accountability and continued improvement, your new product development process will continue to evolve and lead to improved results now and in future.
