There is increasing public concern about the presence of endocrine disruptors in consumer products. This preoccupation requires manufacturers to be more transparent and to provide more precise information to consumers.
Toxicologists in charge of safety assessment files must now incorporate this parameter into their analysis. The IDEA TESTS GROUP, in partnership with LABORATOIRE WATCHFROG, a reference laboratory for the measurement of endocrine disruptors, proposes 3 alternative tests on major endocrine axes, in compliance with current regulations.
Measure the adverse effects of your products for regulatory schedules
On June 2016, the European Commission released a proposal to define endocrine disruptors. The chosen definition is based on that of the World Health Organisation: "an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations"(1). This definition will be included in all current and forthcoming European regulations, in particular the European Cosmetic Regulation.
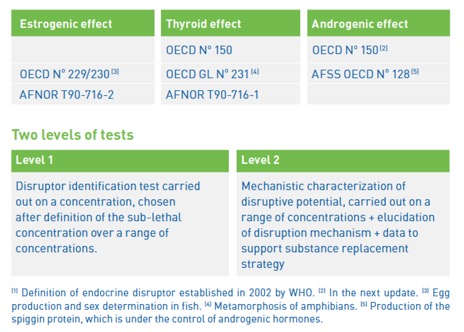
The IDEA TESTS GROUP and LABORATOIRE WATCHFROG offer 3 tests to analyse the endocrine disruptors effects in accordance with the criteria outlined in the following OECD guidelines:
The method
The method is performed on aquatic organisms carrying a potential endocrine disruption marker gene promoter, coupled to a reporter gene of fluorescence. The fluorescence level detected is directly proportional to endocrine disruption.
Depending on the result, the product is characterised by a scale of disturbance (Level 0: product without effect at Level 3: known perturbator).The results are also expressed in the form of hormone equivalent.
The Benefits
- Study of disruptive effects on the scale of the organism.
- Real alternative to animal testing : Larvae and fry at 'post-egg' stages (D0 to D8) prior to developmental stages considered as laboratory animals
- Quick method: results under 7 days for a level 1 test.
- Easy results interpretation : physiological disturbance criteria defined in accordance with the OECD.
For more information, contact
M. Frédéric NUNZI, PhD, Doctor in cell biology, Eurotox Expert
+33 (0)5 56 64 82 33
f.nunzi@groupeideatests.com



